42 fda approved health claims on food labels
Health Claims on Food Labels | Cigna The health claims must be balanced and based on current, reliable scientific studies and must be approved by the U.S. Food and Drug Administration (FDA). Health claims may be statements like "This food is a good source of calcium. Adequate intake of calcium may reduce the risk of osteoporosis," or "Development of cancer depends on many factors. Health Claims on Food Labels | Kaiser Permanente The health claims must be balanced and based on current, reliable scientific studies and must be approved by the U.S. Food and Drug Administration (FDA). Health claims may be statements like "This food is a good source of calcium. Adequate intake of calcium may reduce the risk of osteoporosis," or "Development of cancer depends on many factors.
› food › food-labeling-nutritionQuestions and Answers on Health Claims in Food Labeling | FDA Health claims in food labeling are claims that have been reviewed by FDA and are allowed on food products to show that a food or food component may reduce the risk of a disease or a health-related ...

Fda approved health claims on food labels
FDA Food Labeling Enforcement - LabelCalc FDA food labeling enforcement. Online nutrition analysis software makes it easy to create FDA-compliant nutrition labels. ... You can then choose from a variety of FDA-approved label formats and sizes to find one that suits your package, ... health claims, or to simply highlight certain nutrients that your product is rich in. Of course, if you ... Code of Federal Regulations Title 21 - Food and Drug Administration The information on this page is current as of Jan 06, 2022. For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Sec. 101.82 Health claims: Soy protein and risk of coronary heart disease (CHD). (a) Relationship between diets that are low in saturated fat and cholesterol and that include soy ... Food labelling and packaging: Nutrition, health claims and ... - GOV.UK Nutrition labelling. You must follow nutrition labelling information rules for all pre-packed products unless both of the following apply: you're a small business with under 10 employees and a ...
Fda approved health claims on food labels. Health Claims on Food Labels: LabelCalc - FDA Compliant Health claims, according to the FDA, are statements about the relationship between a food product or ingredient and a reduced risk of disease or a health condition. Basically, the FDA distinguishes two kinds of health claims: "authorized" and "qualified.". Authorized Health Claims: Claims that have significant scientific agreement (SSA). Food Labeling and Qualified Health Claims | Mercatus Center The FDA is seeking comment on different approaches for regulating qualified health claims on conventional human food labels and dietary supplements.SummaryThe Nutrition Labeling and Education Act of 1990 gave the Food and Drug Administration (FDA) authority to permit health claims on food labels. A "health claim" is any claim that a substance in the food affects disease or other health conditions. › food › food-labeling-nutritionQualified Health Claims | FDA Mar 07, 2022 · Food manufacturers can petition the agency to consider exercising enforcement discretion for the use of a qualified health claim. The FDA does not “approve” qualified health claim petitions. Health Claims on Food Labels - Kaiser Permanente Topic Overview. Food makers can make health claims about certain nutrients, such as calcium, fiber, and fat, that are found naturally in foods. The health claims must be balanced and based on current, reliable scientific studies and must be approved by the U.S. Food and Drug Administration (FDA). Health claims may be statements like "This food ...
Food Packaging Claims | American Heart Association There are three categories of claims defined by statute and/or FDA regulations that can be used on food and dietary supplement labels: health claims, nutrient content claims, and; structure/function claims. A "health claim" by definition has two essential components: A substance (whether a food, food component, or dietary ingredient) and Health Claims on Food Labels - Consumer Reports Specifically, grass-fed meat and dairy has a more healthful ratio of omega-6 polyunsaturated fatty acids to omega-3s. Too much omega-6 fat in your diet can cause inflammation, which may be a ... Health Claims on Food Labels | LegalMatch A health claim involves whether or not some type of food or food component can affect a disease or health-related condition. In other words, a health claim can be a label on a product that says how the food is beneficial in helping to prevent or treat some kind of health condition. For example, your orange juice container may have a health ... Is It Really 'FDA Approved?' FDA is responsible for protecting public health by regulating human drugs and biologics, animal drugs, medical devices, tobacco products, food (including animal food), cosmetics, and electronic ...
Understanding Health Claims on Food Labels - Food Smart Colorado There are a total of 12 health claims approved by the FDA. A complete listing of health claims approved for food labels is available here: #2- Nutrient content claims describe a food and the level of a particular nutrient in that food. "Low fat," and "High fiber" are both examples of nutrient content claims. For a table showing nutrient ... Qualified Health Claims — FDA Reader A Qualified Health Claim is a statement approved by the FDA for use on food labels that has strict wording requirements. When there is emerging evidence between a food and the reduced risk of a disease or health condition, but not enough for the FDA to issue an Authorized Health Claim, the FDA may approve a "Qualified Health Claim". Health Claims on Food Labels | PeaceHealth The health claims must be balanced and based on current, reliable scientific studies. And the claims must be approved by the U.S. Food and Drug Administration (FDA). Health claims may be statements like: "This food is a good source of calcium. Adequate intake of calcium may reduce the risk of osteoporosis." Pet Food | FDA Feb 04, 2022 · For more information about labeling requirements, see Pet Food Labels - General. FDA also reviews specific claims on pet food, such as “maintains urinary tract health,” “low magnesium ...

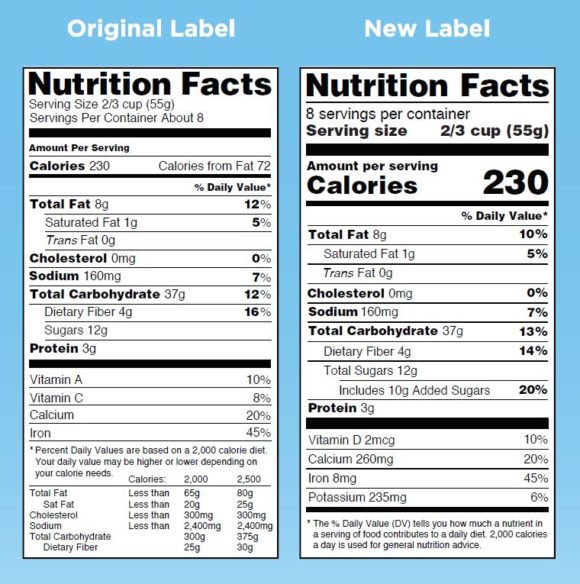
The FDA is making a big change to nutrition labels. And it’s probably a big mistake. | Nutrition ...
A Guide to FDA Regulation of Food Labeling Claims Among the FDA-regulated claims commonly declared on food labels are nutrient-content claims, health claims, qualified health claims and structure/function claims. Additionally, FDA has authority over claims related to gluten content, genetically modified organisms (GMOs) and "natural." It is critical that companies familiarize themselves with ...
Is CBD Oil Approved by FDA - May 2022 - CBD Clinicals May 01, 2022 · Last year, the FDA sent out warning letters to several CBD manufacturers making unsubstantiated claims regarding the therapeutic uses of cannabidiol. (21) This type of practice is what the FDA remains concerned with, and they plan to take action against small businesses that continue to do so.
Code of Federal Regulations Title 21 - Food and Drug Administration For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Sec. 101.14 Health claims: general requirements. (a) Definitions. For purposes of this section, the following definitions apply: (1) Health claim means any claim made on the label or in labeling of a food, including a dietary supplement ...
Dietary Supplements - Claims and Labeling Compliance for FDA/FTC Legal advice concerning FDA-compliant labeling, including regulation of: Nutrient Content Claims; Structure-function claims; Health Claims; Qualified health claims; Organic claims; Legally compliant labeling including label information regarding 'supplement facts' Legal review of website, third-party literature, advertising, and marketing content
Nutrient Content Claim vs Health Claim - LabelCalc Nutrient content claims, which are commonly used on food labels, either refer to the amount of a nutrient in a product or compare the levels of a nutrient in that food to a similar reference food. ... According to the FDA, health claims refer to the relationship between a specific food product or ingredient and a reduced risk of disease or a ...
Are the health claims on food labels accurate and reliable? The health claims must be balanced and based on current, reliable scientific studies and must be approved by the U.S. Food and Drug Administration (FDA). Health claims may be statements like "This food is a good source of calcium. Click to see full answer. Similarly, you may ask, what health claims are allowed on food labels?
Legal Guide to Health Claims on Food | Law@Dayton The Nutrition Labeling and Education Act, which amended the FD&C Act in 1990, requires most foods to be labeled with serving sizes and specific nutrition information, and it sets standards for food labels that make certain health claims. The Fair Packaging and Labeling Act of 1966 spells out packaging requirements for food and other packaged ...
› food › food-labeling-nutritionLabel Claims for Food & Dietary Supplements | FDA Mar 07, 2022 · Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims,...
What are some examples of an FDA health claim on a food label? A diet low in total fat may reduce the risk of some cancers." But just because a food label has a health claim does not mean that the food is healthy for you. For example, a food that is labeled as "a good source of calcium" may still be high in fat, salt, or sugar. What are the two types of claims on food labels that are regulated by the FDA?

Ishigaki Glutathione Now FDA Approved | Dear Kitty Kittie Kath- Top Lifestyle, Beauty, Mommy ...
Introduction to Food Product Claims — FDA Reader A Qualified Health Claim is a statement approved by the FDA for use on food labels that has strict wording requirements. When there is emerging evidence between a food and the reduced risk of a disease or health condition, but not enough for the FDA to issue an Authorized Health Claim, the FDA may approve a "Qualified Health Claim".
› food › food-labeling-nutritionAuthorized Health Claims That Meet Significant Scientific ... Authorized health claims in food labeling are claims that have been reviewed by FDA and are allowed on food products or dietary supplements to show that a food or food component may reduce the ...
Health Claims On Your Food - Tufts Health & Nutrition Letter "If it is indeed an FDA-approved health claim, consumers can be reassured that there is scientific evidence to warrant putting the claim on the package." Such health claims have a standard format you can learn to identify. ... A Food Labeling Guide (Claims) To learn more: Critical Reviews in Food Science and Nutrition, online November 2015.
Label Claims for Conventional Foods and Dietary Supplements | FDA Appendix C of The Food Labeling Guide contains a summary of those health claims that have been approved for use on food and dietary supplement labels. A Food Labeling Guide - Appendix C: Health ...
Factual Food Labels: Health Claims - University of Texas at Austin According to the United States Food and Drug Administration (FDA) there are only three categories of claims that are approved to be printed on food packaging: health claims, nutrient claims, and function claims. Generally, these labels are found on the front side of the food package in emphasized lettering. Health Claims. In 1990, the Nutrition ...
Food labelling and packaging: Nutrition, health claims and ... - GOV.UK Nutrition labelling. You must follow nutrition labelling information rules for all pre-packed products unless both of the following apply: you're a small business with under 10 employees and a ...
Code of Federal Regulations Title 21 - Food and Drug Administration The information on this page is current as of Jan 06, 2022. For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Sec. 101.82 Health claims: Soy protein and risk of coronary heart disease (CHD). (a) Relationship between diets that are low in saturated fat and cholesterol and that include soy ...
FDA Food Labeling Enforcement - LabelCalc FDA food labeling enforcement. Online nutrition analysis software makes it easy to create FDA-compliant nutrition labels. ... You can then choose from a variety of FDA-approved label formats and sizes to find one that suits your package, ... health claims, or to simply highlight certain nutrients that your product is rich in. Of course, if you ...










Post a Comment for "42 fda approved health claims on food labels"