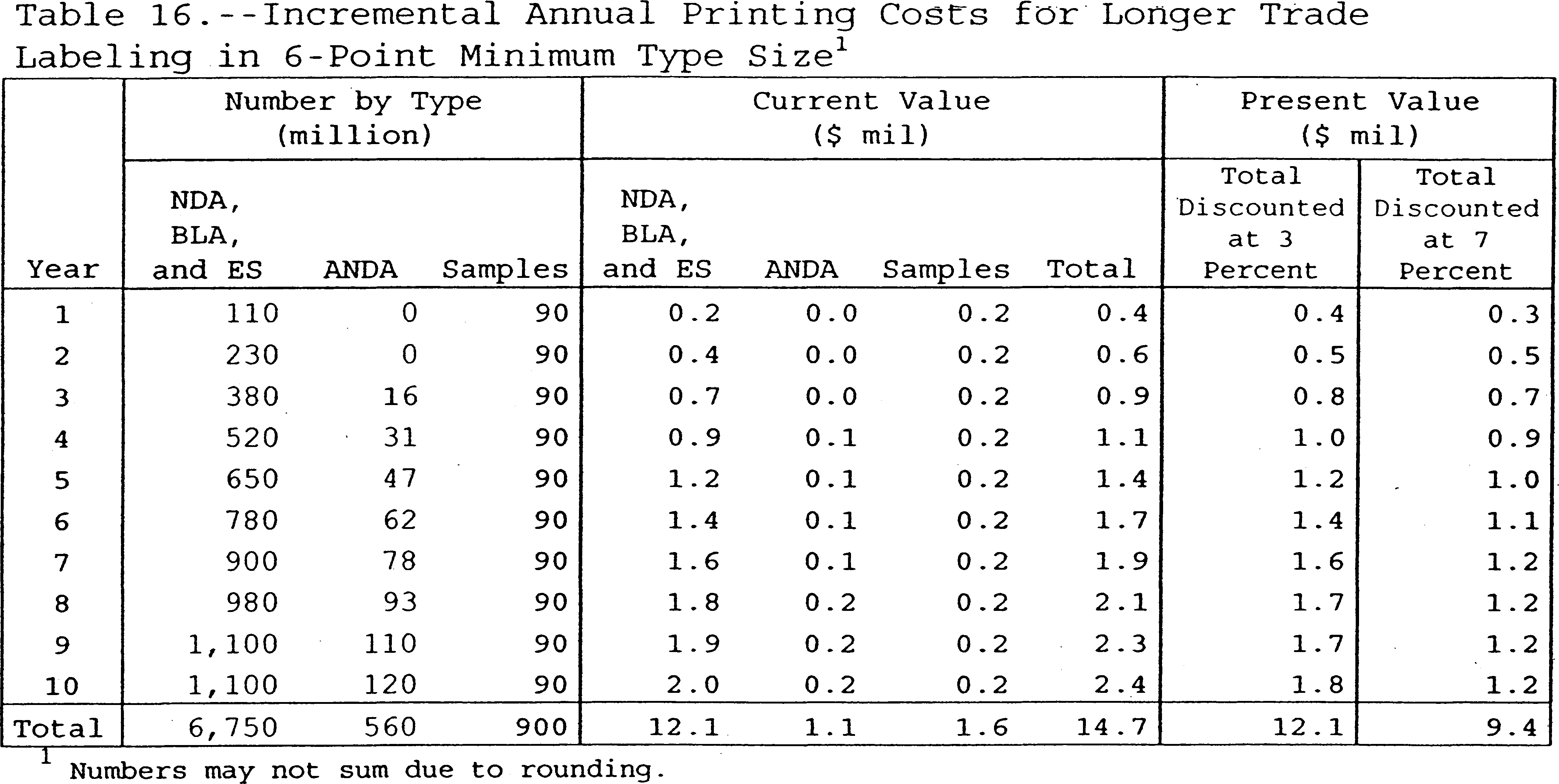
39 auxiliary labels drug reference chart
Auxiliary Labels | Nev's Ink AUXILIARY LABEL, PRESCRIPTION CAN BE REFILLED - PAPER - PERMANENT - 3/8" X 1-1/2" - YELLOW W/BLACK - 1,000 - ROLL PAUXW-0013 $8.18 AUXILIARY LABEL, REFILLED BY AUTHORITY OF YOUR DOCTOR - PAPER - PERMANENT - 3/8" X 1-1/2" - WHITE W/BLACK - 1,000 - ROLL PAUXW-0011 $8.18 Pharmacy Tech. : Auxiliary Label Chart Flashcards - Quizlet Pharmacy Tech. : Auxiliary Label Chart STUDY Flashcards Learn Write Spell Test PLAY Match Gravity Created by mattvah Terms in this set (43) ACE Inhibitor -May Cause Dizziness -Avoid Alcohol -Take on empty stomach ADHD -Avoid caffeine -May cause insomnia -May be habit-forming Analgesic -May cause dizziness -May cause drowsiness Antibiotic
PDF Chapter 24 Medication Administration (Charting, Documentation and The ... When a PRN medication is administered, the nurse should properly document in the chart the following: a. The complaint or the symptom for which the drug was given. b. The dose, time, route of administration, and if appropriate the site of the injection. c. The results achieved or the statement no results achieved . d. The nurse's signature.

Auxiliary labels drug reference chart
Veterinary Auxiliary Labels - Nev's Ink AUXILIARY LABEL, PRESCRIPTION CAN BE REFILLED - PAPER - PERMANENT - 3/8" X 1-1/2" - YELLOW W/BLACK - 1,000 - ROLL PAUXW-0013 $8.18 AUXILIARY LABEL, GENERIC DRUG HAS BEEN DISPENSED AT LOWER PR - PAPER - PERMANENT - 3/8 - RED W/BLACK - 1,000 - ROLL PAUXW-0012 $8.18 Labels and Tape | United States - Nev's Ink Nev's Ink provides companies with high-quality labeling and tape solutions tailored to fit all of your business’s needs. Our state-of-the-art printing facility allows us to provide high quality, cost-effective solutions with some of the fastest turn times in the industry. 49 Pa. Code Chapter 27. State Board Of Pharmacy (5) The dispensing pharmacist, the prescribing or administering practitioner, the cancer drug repository, the Board and any other participant of the Cancer Drug Repository Program cannot guarantee the safety of the drug being dispensed or administered, and that the pharmacist has determined that the drug appears to be safe to dispense or administer based on the accuracy …
Auxiliary labels drug reference chart. PDF Pharmaceutical Compounding and Dispensing - Ducopharm important additional auxiliary labels. The summary list given in Table 1.1 is to be used as a guide in the absence of any guidance from the offi cial pharmaceutical texts. It should be remembered that the information in this table is to be used only as a guide. Any information on additional labelling or expiry dates in the offi cial texts Simple Strategies to Avoid Medication Errors - American Academy … In any given week, four out of five U.S. adults will use prescription medicines, over-the-counter drugs, or dietary and herbal supplements. Nearly one-third of adults take five or more different ... Guidance Document: Labelling of Pharmaceutical Drugs for Human Use 3.8 Labelling of Professional Samples 3.9 Including International Information on Drug Package Labels Claims and Text Content 4.1 Misrepresentation of Classification 4.2 Absence of Ingredients 4.2.1 Sugar-free, Sucrose-free, Sweetener-free 4.2.2 Salt and Sodium-free 4.3 Absence of Side Effects 4.4 Side Effects and Placebo Comparisons PDF COMPLIANCE LABEL AND DRUG REFERENCE CHART • Ri 361 Steelcase Road West ... COMPLIANCE LABEL AND DRUG REFERENCE CHART • Ri 361 Steelcase Road West, unit Markham, Ontario L3R 3V8 Toronto 905-47 S -2500 Ton Free: :RTOIRE
Code of Laws - Title 40 - Chapter 43 - South Carolina ... SECTION 40-43-10. Short title; purpose of chapter; severability. This chapter may be cited as the "South Carolina Pharmacy Practice Act". The purpose of this chapter is to promote, preserve, and protect the public health, safety, and welfare by and through the effective control and regulation of the practice of pharmacy; the licensure of pharmacists; the licensure, permitting, control, and ... eCFR :: 10 CFR Part 430 -- Energy Conservation Program for … Cold temperature fluorescent lamp means a fluorescent lamp specifically designed to start at −20 °F when used with a ballast conforming to the requirements of ANSI C78.81 (incorporated by reference; see § 430.3) and ANSI C78.901 (incorporated by reference; see § 430.3), and is expressly designated as a cold temperature lamp both in markings on the lamp and in … Guidance Document : Post-Notice of Compliance (NOC) Changes ... a sample of the inner and outer labels (Level I changes require label mock-ups while Level II changes require written text in place of mock-ups) to reflect any proposed changes. (d) An annotated and non-annotated electronic copy of the relevant Health Canada Quality Overall Summary template (QOS), or the revised sections of the QOS. Browse Jobs - Key Personnel General Shop Helper - Cutting Table Unload Pay: $14.00 Hours: 1st shift 6:00pm-6:00am, Monday through Friday Catoosa Job Description: • Efficiently unload glass from Cutting Table • Properly sort glass to the appropriate racks • Transport glass from one location to another by carrying or using appropriate machinery • Read and attach appropriate labels to products • …
49 Pa. Code Chapter 27. State Board Of Pharmacy (5) A statement identifying the terms under which a pharmacist providing the management of drug therapy is permitted to: adjust the drug regimen, the drug strength and the frequency of administration or the route of administration; administer drugs; order laboratory tests; and order and perform other diagnostic tests necessary in the management ... PDF Chapter 20 Labeling Medications and Expiration Dating and location, directions for use, and auxiliary labels c. Other labeling considerations: 1) The initials of the person preparing and verifying each compound 2) Placement of labels a) Affixed to containers so that they may be read while hanging b) Avoid covering manufacturer labeling containing drug name, Guidance Document : Post-Notice of Compliance (NOC) Changes: … (P.8.2) Where the route of synthesis of the drug substance has been substantially changed or for drug products where stability issues are known (e.g. due to poorly stable drug substances) or where the changes may affect manufacturing or performance of the drug product, an updated post-approval stability protocol and stability commitment to place the first commercial scale … Labeling Information | Drug Products | FDA For more information on labeling, including Physician Labeling Rule (PLR) requirements, guidances, presentations, sample templates and format tools, and established pharmacologic class (EPC ...
Comparison: China and United States | ClinRegs All entities or drug research institutions holding drug marketing authorizations must take responsibility for drug safety, effectiveness, and quality controllability in the whole process of drug research and development, production, distribution, and use. Based on this system, the MAHs are also named as applicants or sponsors during clinical ...
2016 Updated American Society of Clinical Oncology/Oncology … Introduction As oncology providers continue to try to improve, the Oncology Nursing Society (ONS) and the American Society of Clinical Oncology (ASCO) have aspired to provide standards to minimize the risk of errors in chemotherapy ordering, preparation, and administration, including both oral1 and parenteral therapy, in both the outpatient2 and inpatient3 settings. …
PDF Guiding Principles for Assigning Auxiliary Labels for Outpatient ... developed the basic guiding principles for assigning auxiliary labels as follows: a. Auxiliary label information enhances but does not replace patient handouts or verbal counselling. b. A maximum of four auxiliary labels will be used due to container size limitation and to avoid alert fatigue. i. Exceptions: additional labels may be affixed to ...
Safer dispensing labels for prescription medicines - NPS MedicineWise The use of standard dosing times, 'morning, noon, evening, bedtime', has been labelled the 'universal medication schedule'. 10, 12 The use of standard dosing times is feasible for most drugs and is less confusing, more informative and makes it easier for patients to consolidate multiple medicines into fewer dosing times throughout the day. 4, 9
PDF Good Label and Package Practices Guide for Prescription Drugs - ISMP Canada Good Label and Package Practices Guide for Prescription Drugs 9 3 Designing Labels and Packages for Safety 3.1 Introduction Part 3 of this guide presents information on current good practices in the design and layout of a health product label, the information contained on the label, and the design or choice of package.
JAMA Network | JAMA Internal Medicine | Improving Prescription Drug Warnings to Promote Patient ...
50 Common Warning Labels On Medication Containers Top 50 Common Warning Labels and Their Meanings The medication must be swallowed whole. Because certain drugs are designed to be either fast-acting or slow-releasing, damaging the outer coating may lead to harmful damages to the body. The medication is intended for external use only. Ingesting it may lead to undesirable effects or even poisoning.
Pharmacy Auxiliary Labels - The Medi-Dose Group (Medi-Dose / EPS / Vu-Pak) All labels measure 3/8" x 1 ½" and are brightly colored so they call attention to themselves. Because these labels have been in use for years, the graphics depicting what's being described are instantly recognized by your patients and practitioners. As always, if you do not find the item you are looking for, please call us at 800-523-8966!
Guidelines for Labeling Pharmaceutical & Healthcare Products Several important things to include on a pharmaceutical or healthcare product label: 3. Formatting Labels for FDA Approval. Your labels must be designed in the appropriate FDA format for your product's classification like OTC medications, oral contraceptives, combination products, etc. Click here for a list of labeling guides relating to drugs.
General Chapter Prescription Container Labeling | USP-NF Estimated proposal PF: 46 (1) Background and objective: General Chapter <17> Prescription Container Labeling provides universal standards for the format, appearance, content, and language of prescription medication instructions to promote patient understanding and reduce medication errors. The Healthcare Quality and Safety Expert Committee ...
PDF BuSpar - Food and Drug Administration any drug's use can be identified only after several years of marketing. Information for Patients To assure safe and effective use of BuSpar, the following information and instructions should be given to patients: 1. Inform your physician about any medications, prescription or non-prescription, alcohol, or drugs that
PDF AM:L30 PRESCRIBING INFORMATION AMOXIL - Food and Drug Administration implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where high dosage of drug can be used. This category also provides a buffer zone, which prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A
PDF Lipitor (atorvastatin calcium) Label - Food and Drug Administration Drug therapy is recommended as an adjunct to diet when the r esponse to a diet restricted in saturated fat and cholesterol and other nonpharmacologic measures alone has been inadequate. In pateinst wtih CHD or mutlpi el rsikfactors for CHD L, IPITOR can be started smi utlaneousyl wtih deit.
Auxiliary Labels | Nev's Ink Our auxiliary labels are brightly colored and use easy-to-read and bold font so there is no chance of miscommunication. ... Drug Dose Labels; Communication Labels; Color-Coding Tape; Color-Coding Labels; Radiology. Mammography Labels; ... Flr Chart w/Black (50) Lavender (49) Gray (47) Dark Green (46) Orange w/Black (45) Dark Blue (42)
Code of Laws - Title 40 - Chapter 43 - South Carolina General … SECTION 40-43-10. Short title; purpose of chapter; severability. This chapter may be cited as the "South Carolina Pharmacy Practice Act". The purpose of this chapter is to promote, preserve, and protect the public health, safety, and welfare by and through the effective control and regulation of the practice of pharmacy; the licensure of pharmacists; the licensure, permitting, …






Post a Comment for "39 auxiliary labels drug reference chart"